Lewis structure of vinyl chloride a vinyl ic halide.
Allylic vs vinylic position.
The allylic carbon atom is more reactive than normal.
Allyl groups have three carbon atoms and five hydrogen atoms.
Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
On or bonded to the carbon of an alkene.
Unlike vinyl group.
Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation.
The allylic carbon is bonded to a carbon atom which is doubly bonded to another carbon atom.
It would be kept mentioned that allyl is the latin word that is used for the garlic allium sativum.
It consists of a methylene bridge ch 2 attached to a vinyl group ch ch 2.
The name is derived from the latin word for garlic allium sativum in 1844 theodor wertheim isolated an allyl derivative from garlic oil and named it schwefelallyl.
An allyl group is a substituent with the structural formula h 2 c ch ch 2 r where r is the rest of the molecule.
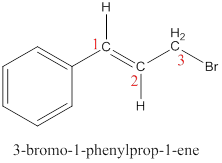
This molecule has four vinyl ic positions each marked with.
Allyl form a stable carbocation because of the electron delocalization whereas vinylic carbocations are unstable as they lack p character.
The key difference between these two structural components is the number of carbon and hydrogen atoms.
The allylic position is also like a vinylic position.
The general formula for allyl is r ch 2 ch ch 2 in which the asterisk carbon atom is an allylic carbon atom.
Allyl have two sp 2 hybridized carbon atoms and one sp 3 hybridized carbon atom.
The key difference between allylic and vinylic carbon is that allylic carbon is the carbon.